Questions? unircell@ua.pt

Fuel Cells and Hydrogen as Energy Vector
Department of Materials and Ceramic Engineering, CICECO – Aveiro Institute of Materials, University of Aveiro, 3810-193 Aveiro, Portugal
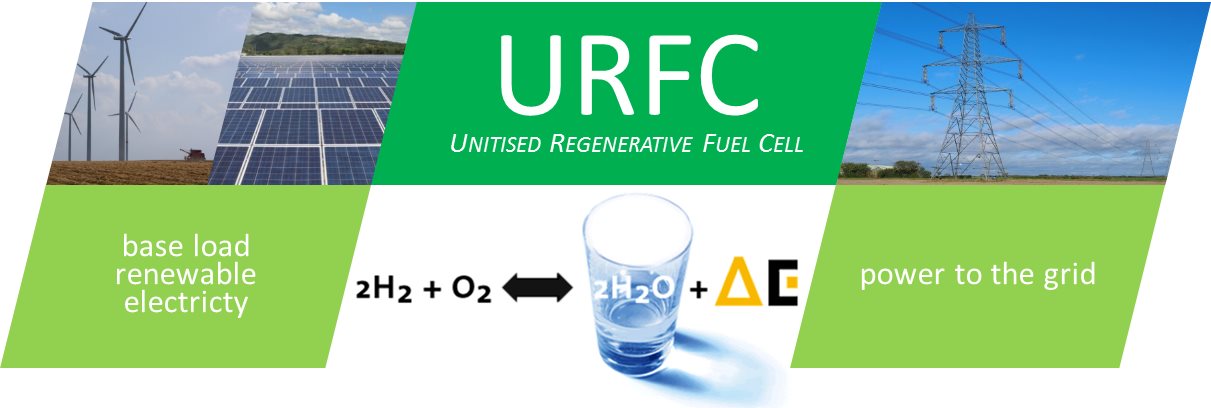
The major drawback of renewable electricity sources like wind or solar is intermittency due to demand and climate conditions. Energy storage is thus a crucial issue for a fully sustainable energy paradigm based on renewable sources of electrical power. (Fig. 1)
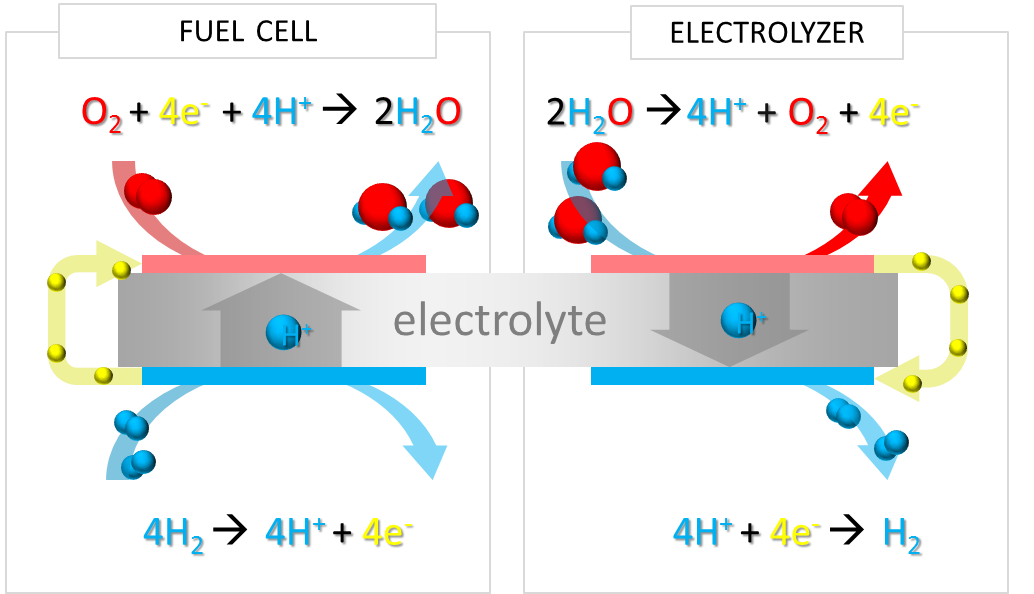
Hydrogen produced by water electrolysis during base load moments, stored and converted back to electricity through the inverse reaction, is the ideal energy vector. The possibility of large-scale use of hydrogen as a transport fuel massively increases the potential for renewables and base-load electricity supply. The Unitized Regenerative Fuel Cell (URFC) including in the same device an electrolyzer (EL) that converts electricity in hydrogen and oxygen, and a Fuel Cell (FC) producing electricity on demand by using the stored hydrogen and oxygen, is an enabler technology (Fig. 2).
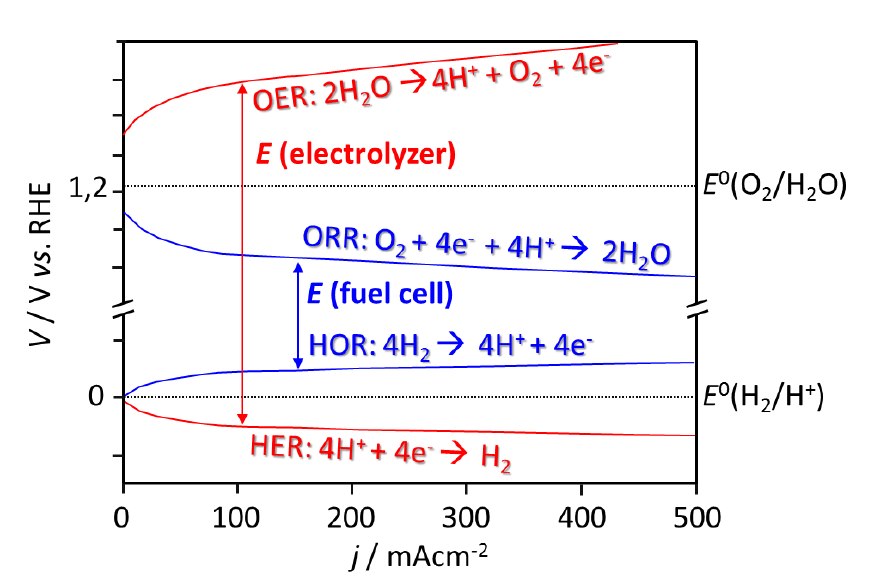
The cost, weight and volume of an URFC are potentially lower than the combination of separate FC and EL units, but the materials and the device critical function requirements are severe (Fig. 3).
The simplification of the thermal and water management systems, the discovery of electrolytes less sensitive to humidity and platinum-free electrocatalysts are key challenges for the URFC technology.
Project UniRCell - Unitised Regenerative Fuel Cell for Efficient Renewable Energy Supply: from Materials to Device aims to develop a new generation of environmentally sustainable, high-performance and low cost materials by demonstrating their application in a prototype URFC.
The work plan is organized in innovation supporting actions and technical activities focused on the:
-development of novel electrolytes;
-development of novel OER/ORR and HER/HOR bifunctional electrocatalysts based on;
-integration of membrane electrode assemblies through evaluation of different electrode configurations, gas diffusion layers and separator plates;
-analytical and experimental critical function/validation of the components in laboratory FC, EL and URFC prototypes.
Modelling approaches are implemented in various activities in order to gain insight on:
-atomistic features of the relationships between structure, ionic transport and electrocatalysis;
-dynamic effects of two-phase flow, heat and mass transfer on device performance optimization.
Funded by COMPETE 2020 and FCT within the framework of the “Programa de Actividades Conjuntas”, the UniRCell research team combines the expertise in ionic conductors from UAveiro/CICECO and UTAD/CQ-VR to develop electrolytes, the materials chemistry from REQUIMTE/LAQV and the surface science from LSRELCM/FEUP acting together on the preparation and evaluation of electrocatalysts, and the transport phenomena engineering of FEUP/CEFT for device integration, testing and optimization.
UniRCell is thus an opportunity to develop and consolidate R&D activities on hydrogen and fuel cells technologies in Portugal, now scarce and lacking critical mass, by proving several training opportunities and scientific jobs.
Fig. 1 / Renewable electricity (wind, solar or hydro) in base load moments is used to produce hydrogen and oxygen through water electrolysis. The produced gases can be stored and subsequently used in a fuel cell to produce electricity, which is fed to the grid upon demand.
This regenerative process can be carried out on a URFC electrochemical reactor.
Fig. 2 / The electrochemical combustion of hydrogen in the fuel cell, and the reverse process in the electrolyzer. Both processes can occur in the same type of electrochemical reactor, comprising a proton conducting electrolyte, electrodes and suitable electrocatalysts.
Fig. 3 / Schematics of the losses in a fuel cell and in a electrolyzer based on a proton exchange membrane operating in acid conditions, where it can be seen that reactions involving hydrogen are more favorable then the reactions involving oxygen. An efficient URFC system implies the development of bifunctional electrocatalysts, i.e., which are able on the one hand to catalyze both REO e RRO, but, on the other hand are also able to improve the kinetics of ROH and REH.
Another key aspect for the URFC technology is the development of electrolytes stable in both oxidizing and reducing conditions under variable and relative humidity conditions.
Copyright © 2019 - UniRCell - Department of Materials and Ceramic Engineering, CICECO – Aveiro Institute of Materials, University of Aveiro. All Rights Reserved.