On the stability in alkaline conditions and electrochemical performance of A2BO4-type cathodes for liquid fuel cells
David S. P. Cardoso, B. Šljukić, N. Sousa, C. A. C. Sequeira,F. M. L. Figueiredo and D. M. F. Santos
Phys.Chem.Chem.Phys., 2018, 20, 19045, doi: 10.1039/c8cp02114g (June 22 2018)
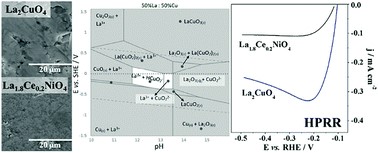
Abstract: Typical direct liquid fuel cells (DLFCs) use a liquid fuel and O2 as the oxidant. However, for applications where O2 is not available (e.g., space and underwater), the gas has been replaced by H2O2 as a liquid oxidant. This work presents a study of various ceramic disc electrodes with K2NiO4 structure and nominal compositions La2NiO4, La2CuO4, La1.9Pr0.1CuO4, La1.9Sr0.1CuO4, La1.8Ce0.2NiO4, La1.9Pr0.1NiO4, La1.8Pr0.2NiO4 and La1.9Sr0.1NiO4 to assess their stability and activity for the hydrogen peroxide reduction reaction (HPRR) in alkaline media. Stability tests conducted in 2 M NaOH show that Ni and Cu are readily dissolved, as occurs for substituting elements such as Sr, in agreement with calculated Pourbaix diagrams. Such degradation affects the surface of the materials, which is depleted of transition metals. This has consequences for the ORR and HPRR activity due to formation of a La-rich passivation layer on the surface. Only La2CuO4 and La1.8Ce0.2NiO4 display HPRR activity at around −0.25 V vs. RHE. An attempt is made to correlate the composition, chemical stability and electrochemical behaviour of these materials based on known molecular-orbital models proposed for the oxygen reduction reaction.
