Electrochemical behaviour of electrogenerated hydrophilic carbon nanomaterials
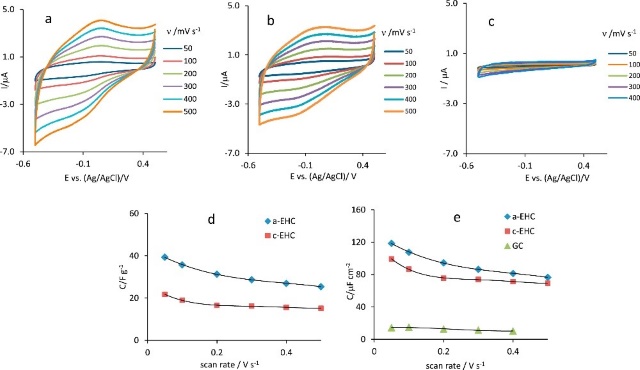
It was recently demonstrated that a highly soluble amorphous carbon based material (EHC-electrogenerated hydrophilic carbon based material) is produced simultaneously at the anode (a-EHC) and cathode (c-EHC) of graphite. In this paper we compare their grade of amorphicity by Raman and their electrochemical properties. For the latter purpose, it was evaluated the potential window, the presence of electrochemically reducible and oxidizable functional groups, the capacitive properties and the electrocatalytic activity towards the oxygen and peroxide reduction reaction in the neutral medium. It was also used Fe(CN)6 3−/4− , Ru(NH3)6 3+/2+ and Fe3+/2+ redox probes to measure the heterogeneous electron transfer (HET) rate. It was found that a-EHC material is the less amorphous material, demonstrating a higher capacitance (120 μF cm−2), higher amount of reducible and oxidizable functional groups, higher ability for the H2O2 reduction (180.7 and 4.3 μA cm−2 mM−1 sensitivity at −1.1 V and at −0.50 V, respectively) and superior catalytic activity for the ORR (17 A g−1 at −0.30 V). Both EHC materials possess a wide potential window and exhibit a HET rate consistent with the surface charge and the different amount/nature of oxygen functionalities on the surface electrode material. This works reveals that EHC materials, in particular a-EHC, show a high potential for a wide range of technological applications in biosensing field and energy conversion and storage devices.
https://doi.org/10.1016/j.electacta.2017.10.197
Autores: R.S. Vieira, A.J.S. Fernandes, M. Cristina Oliveira