Oxidised carbon nanotubes were subjected to a controlled thermal treatment at different temperatures under a N2 atmosphere. X-ray photoelectron spectroscopy confirmed the presence of acid and basic groups on the starting sample.
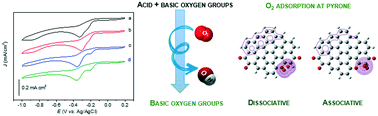
For samples subjected to higher temperatures, only the basic oxygen functional groups remained on the surface, namely, the quinone and pyrone groups. The oxygen reduction reaction (ORR) performance of all the electrocatalysts was evaluated under alkaline medium in O2-saturated solutions. Increasing the basic character leads to better ORR activity in terms of potential onset values (from −0.230 to −0.194 V). Two well-defined cathodic peaks associated with the reduction of oxygen are observed in the cyclic voltammograms when the acid oxygen groups are removed from the electrocatalyst surface. The computational simulations of a carbon surface functionalized with quinone and pyrone groups were a relevant tool to explain the presence of the two cathodic peaks. The quinone sites only favour the adsorption of oxygen by the associative process, while oxygen adsorption on the pyrone group may occur by dissociative and associative processes, involving high electron densities. Consequently, pyrone may require less energy to initiate the ORR mechanism, resulting in a less negative cathodic peak potential value compared with that of the peak of the quinone group.
http://dx.doi.org/10.1039/C7CY00020KRef. Completa do artigo: I. Rocha, O.S.G.P. Soares, J.L. Figueiredo, C. Freire. M.F.R. Pereira, “Bifunctionality of the pyrone functional group in oxidized carbon nanotubes towards Oxygen Reduction Reaction”, Catalysis Science & Technology, 7 (2017) 1868-1879.
